Co Nh2 2 Molar Mass
If we have a chemical compound like NaCl the molar mass will be equal to the molar mass of one atom of sodium plus the molar mass of one atom of chlorine. Carbon2427 12 202.

How To Find The Number Of Atoms In Co Nh2 2 Urea Youtube
Now we have to divide all the values with the lowest obtained value.
. Nitrous acid is also highly volatile in the gas phase it exists predominantly as a trans-planar moleculeIn solution it is unstable with respect to the disproportionation reaction. HNO 2 H NO 2. Create an equation for each element C O H where each term represents the number of atoms of the element in each reactant or product.
To Calculate Empirical Formulae first we have to divide the given percentages of atoms by their molecular masses. 3 HNO 2 aq H 3 O NO 3 2 NO. Centred Broto-Moreau autocorrelation of lag 4 weighted by mass.
B Find the velocity components u r and u θ at any point. In a molecular formula we describe the number of atoms of all the elements presen. Thermosetting polymers- initial mixture of reactive low molar mass compounds reacts upon heating in the mold to form an insoluble infusible network.
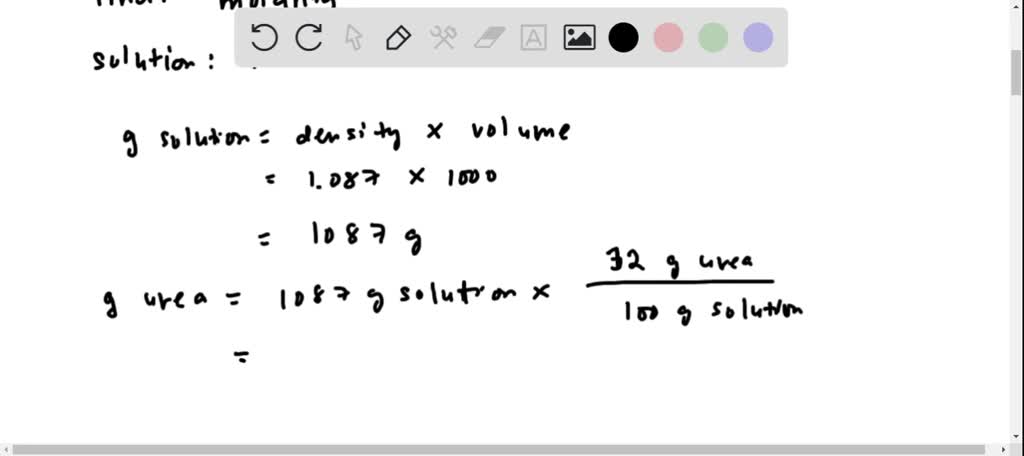
If we write this as a calculation it looks like this. Frequency characteristics and risk factors Voigtlaender et al. Molar Mass Is 9896G.
Anthropogenic MACCity GFAS biomass burning and only to a. Hydrogen 407 201 2. CH3 CO2 2.
Centred Broto-Moreau autocorrelation of lag 2 weighted by mass. Create a System of Equations. Determine the molecular formula of a compound that has a molar mass of 1832 gmol and an empirical.
A A b B Product Rate α Aa Bb Rate K Aa Bb Order of reaction sum of exponents Order of A a and B b Then Overall order a b. Molar masses of chemical compounds are equal to the sums of the molar masses of all the atoms in one molecule of that compound. Because of its relatively short lifetime NO2 in the CAMS reanalysis is largely affected by the prescribed emissions eg.
Centred Broto-Moreau autocorrelation of lag 3 weighted by mass. Find here online price details of companies selling Urea. The molar CO 2 N ratio is usually considered as the amine.
2 a 1 b 6 c. Zeman and co-workers in their system showed how significant the water loss can be with an average mass loss of 90 g of H 2 Og of CO 2 captured. A CO 2 b H 2 O c C 6 H 12 O 6 d O 2.
C Find ψ and sketch a few streamlines. The maximum CO 2 N ratio achievable by chemical reaction with amine sorbents under such conditions is 05 mol of CO 2 mol of N. Get info of suppliers manufacturers exporters traders of Urea for buying in India.
Given u r 1r and u θ 1r find the stream function ψ and sketch the flow field. Hydrogen 407 1 407. Types of polymerization 1.
P_VSA-like on Molar Refractivity bin 3. 1 a 0b 6 c 0d O. Please note that a conversion of the mass mixing ratios kgkg to volume mixing rations molar fractions molmol is needed to do this in a meaningful way.
A Sketch lines of constant φ. PK a 33 at 18 C. This reaction is slow at 0 C.
An irrotational incompressible velocity field is described by the velocity potential φ A θ A 0. Chlorine7165 355 201. Nitrite is the conjugate base of the weak acid nitrous acid.
Law of mass action The rate of a reaction is proportional to the molar concentrations of the reactants each raised to power equal to the number of molecules undergoing reaction. Bakelite is formed of phenol and form-aldehyde polymerization. Thromboembolic events in deceased patients with proven SARS-CoV-2 infection.
P_VSA-like on Molar Refractivity bin 2.

Determine The Molality Of Urea Co Nh2 2 Molar Mass Itprospt

Calculate Relative Molecular Mass For Co Nh2 2 Brainly In

Calculate Molar Mass Of Following Compounds I Co Nh2 2 Ii Ch3coch3 Iii H3bo3 Iv H2so4 Youtube

No comments for "Co Nh2 2 Molar Mass"
Post a Comment